PDF) Aminophosphine Palladium Pincer-Catalyzed Carbonylative Sonogashira and Suzuki–Miyaura Cross-Coupling with High Catalytic Turnovers

Aminophosphine Palladium Pincer-Catalyzed Carbonylative Sonogashira and Suzuki-Miyaura Cross-Coupling with High Catalytic Turnovers. - Abstract - Europe PMC

Dichloro‐Bis(aminophosphine) Complexes of Palladium: Highly Convenient, Reliable and Extremely Active Suzuki–Miyaura Catalysts with Excellent Functional Group Tolerance - Bolliger - 2010 - Chemistry – A European Journal - Wiley Online Library
![Mizoroki-Heck Cross-coupling Reactions Catalyzed by Dichloro{bis[1,1',1''-(phosphinetriyl)tripiperidine]}palladium Under Mild Reaction Conditions | Protocol Mizoroki-Heck Cross-coupling Reactions Catalyzed by Dichloro{bis[1,1',1''-(phosphinetriyl)tripiperidine]}palladium Under Mild Reaction Conditions | Protocol](https://cloudfront.jove.com/files/ftp_upload/51444/51444fig3.jpg)
Mizoroki-Heck Cross-coupling Reactions Catalyzed by Dichloro{bis[1,1',1''-(phosphinetriyl)tripiperidine]}palladium Under Mild Reaction Conditions | Protocol

Multifunctional chiral aminophosphines for enantiodivergent catalysis in a palladium‐catalyzed allylic alkylation reaction - Eliseenko - 2020 - Chirality - Wiley Online Library

Origins of the enantioselectivity of a palladium catalyst with BINOL–phosphoric acid ligands - Org. Biomol. Chem. - X-MOL

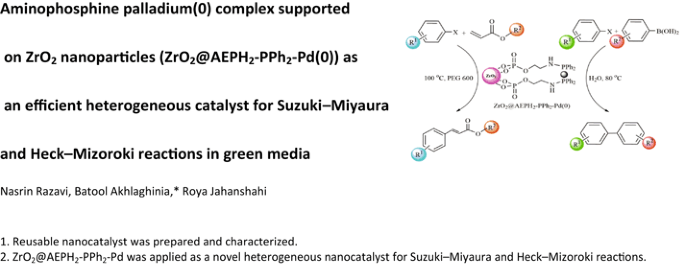
Table 1 from Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Semantic Scholar
![Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022328X07001738-fx1.jpg)
Synthesis and characterization of palladium(II) complexes with chiral aminophosphine ligands: Catalytic behaviour in asymmetric hydrovinylation. Crystal structure of cis-[PdCl2(PPh((R)-NHCHCH3Ph)2)2] - ScienceDirect

PDF) Aminophosphine–palladium(II) complexes: Synthsesis, structure and applications in Suzuki and Heck cross-coupling reactions | Murat Aydemir - Academia.edu
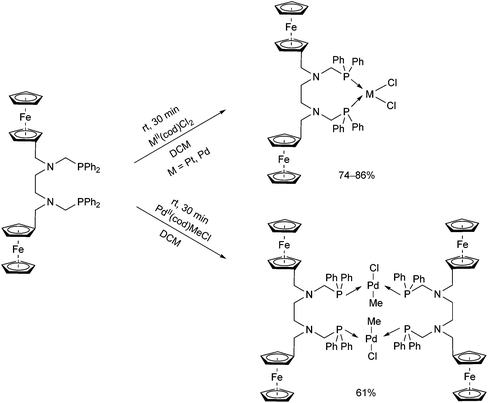
Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/C8DT00178B
![The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ... The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...](https://onlinelibrary.wiley.com/cms/asset/734bf342-fa45-4f08-a712-c34ceb2581a0/msch001.jpg)
The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...
![The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ... The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...](https://onlinelibrary.wiley.com/cms/asset/16d0c16a-ffaf-4992-b9e6-91a5da65039b/mcontent.jpg)
The 1,3‐Diaminobenzene‐Derived Aminophosphine Palladium Pincer Complex {C6H3[NHP(piperidinyl)2]2Pd(Cl)} – A Highly Active Suzuki–Miyaura Catalyst with Excellent Functional Group Tolerance - Bolliger - 2010 - Advanced Synthesis & Catalysis - Wiley ...

Camphane-based aminophosphine ligands for Pd-catalyzed asymmetric allylic alkylation - ScienceDirect

Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Request PDF
Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/C8DT00178B

Synthesis, structure, computational and catalytic activities of palladium complexes containing hydrazide based amino-phosphine ligands - ScienceDirect
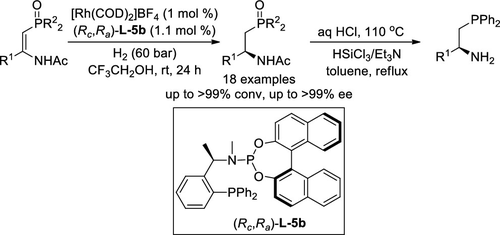
Rh-Catalyzed Asymmetric Hydrogenation of (Z)-β-Phosphorylated Enamides: Highly Enantioselective Access to β-Aminophosphines. - Org. Lett. - X-MOL

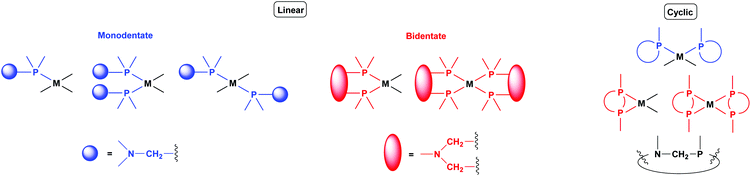
Bis-N,N-aminophosphine (PNP) crosslinked poly(p-tert-butyl styrene) particles: A new support for heterogeneous palladium catalysts for Suzuki coupling reactions - Catal. Commun. - X-MOL

Table 1 from Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Semantic Scholar

Table 1 from Aminophosphine Palladium(0) Complex Supported on ZrO2 Nanoparticles (ZrO2@AEPH2-PPh2-Pd(0)) as an Efficient Heterogeneous Catalyst for Suzuki–Miyaura and Heck–Mizoroki Reactions in Green Media | Semantic Scholar
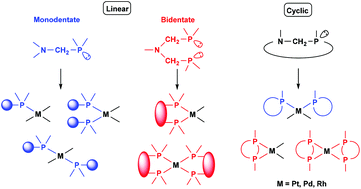
Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands - Dalton Transactions (RSC Publishing)