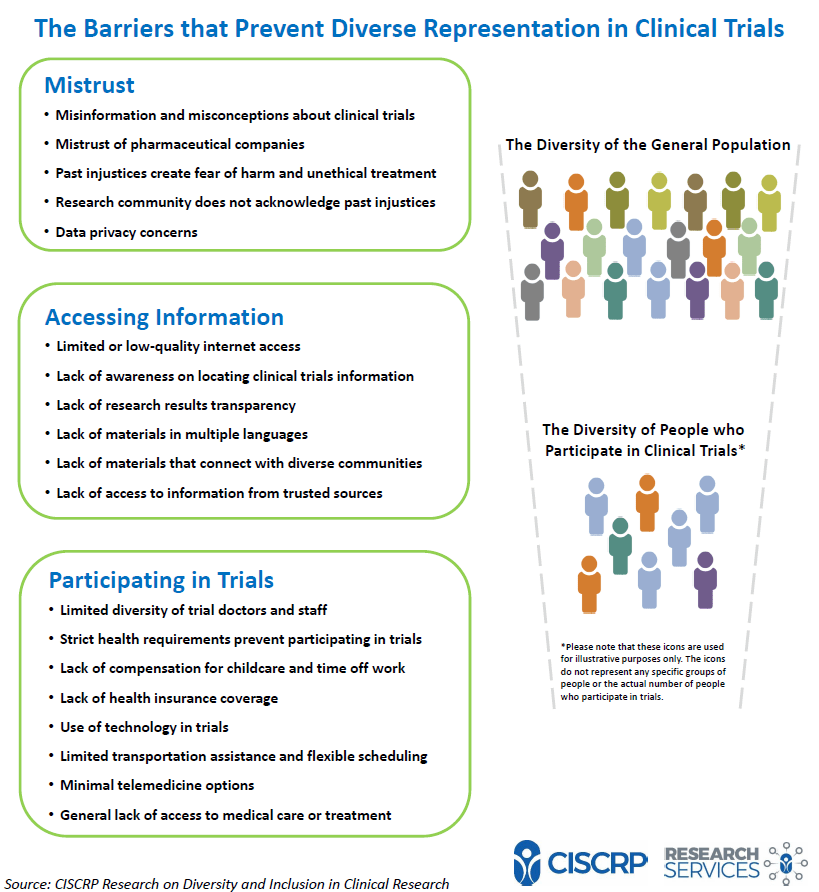
Doing Our Part: Improving Diversity in Clinical Research Participation - Center for Information & Study on Clinical Research Participation

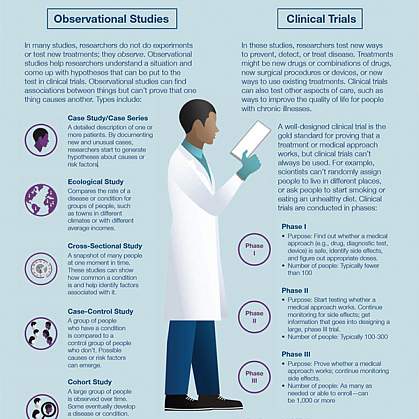
A Guide to Understanding Clinical Trials: Part 2 - Five Factors to Consider When Evaluating Results - Bench Press

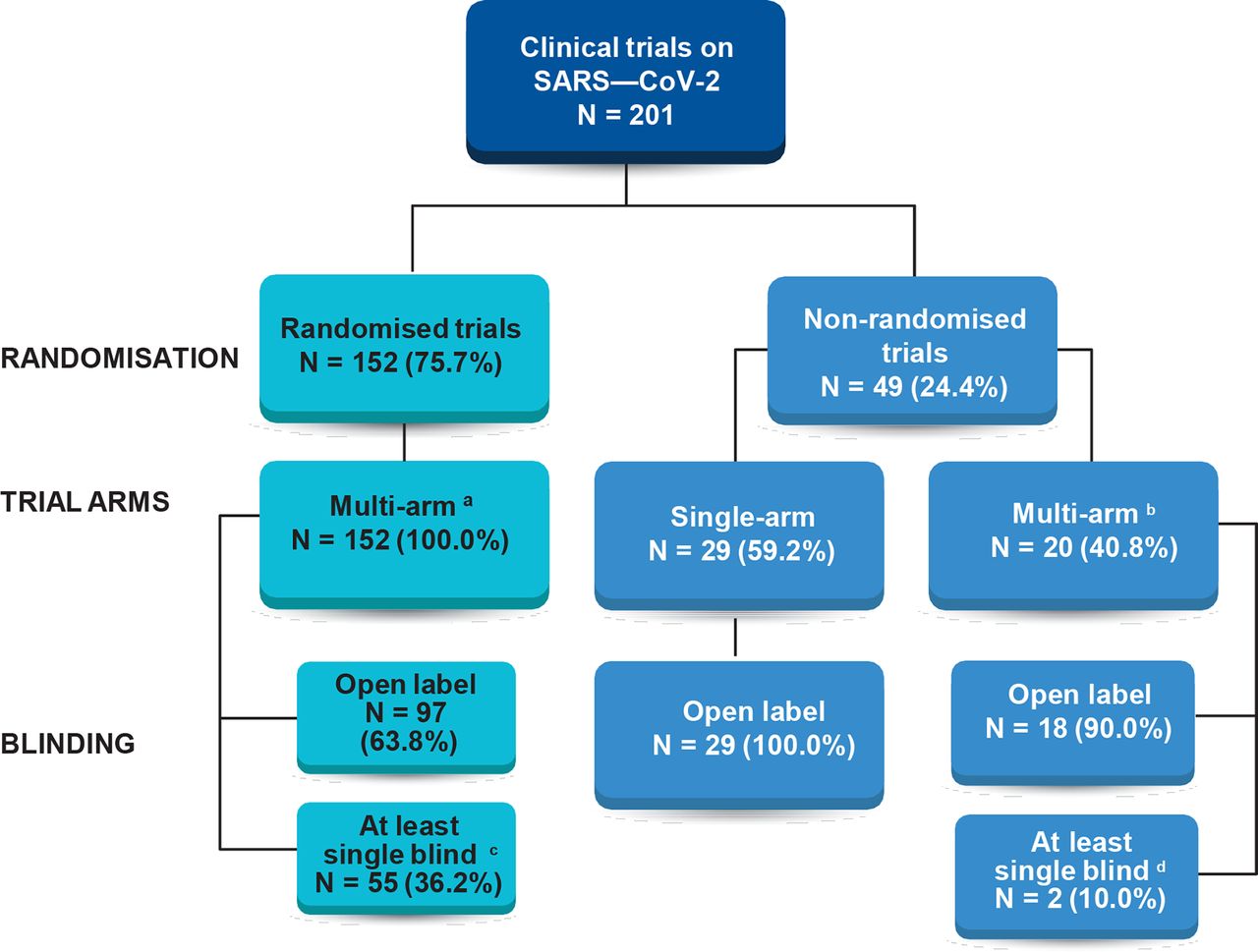
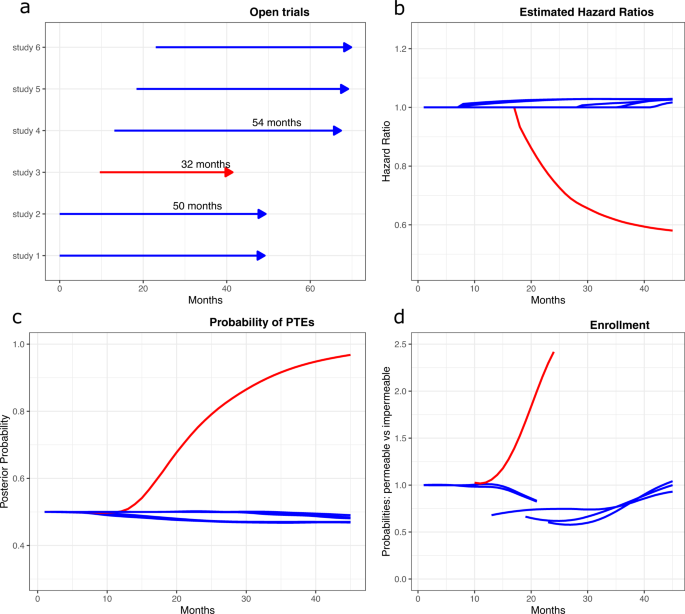
Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology

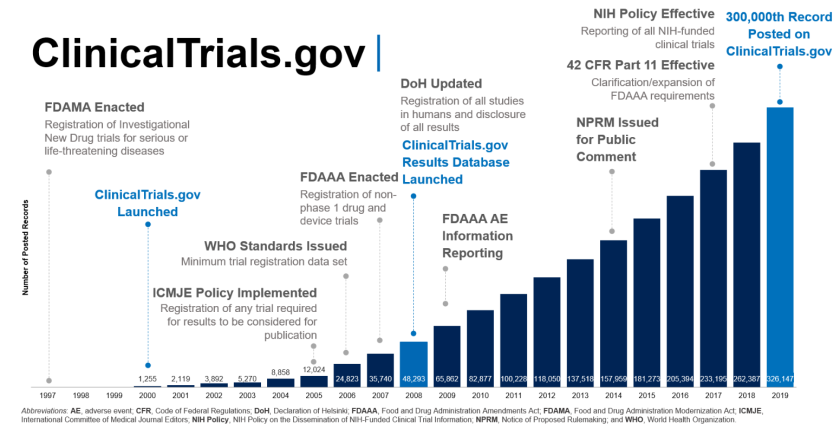
Final Rule Confirms, Posting of Study Results on ClinicalTrials.gov Will be Required for Unapproved Products - IMPACT Pharmaceutical Services, Inc.
How Frequently Do the Results from Completed US Clinical Trials Enter the Public Domain? - A Statistical Analysis of the ClinicalTrials.gov Database | PLOS ONE